Pressure is a fundamental concept in the world of physics and engineering, and it is crucial in various applications, from fluid mechanics to meteorology and medical science. Pressure is typically measured in different units depending on the context, and one of the widely used units is millimeters of mercury (mmHg). In this article, we will explore what mmHg is, how it relates to other pressure units, and how to calculate pressure in mmHg.
What Is mmHg?
Millimeters of mercury (mmHg) is a unit of pressure that measures the height of a column of mercury supported by atmospheric pressure. The concept of mmHg dates back to the early days of mercury barometers, which were used to measure atmospheric pressure. In a mercury barometer, a column of mercury is placed in a vertical glass tube, and the height to which the mercury rises in the tube is a direct measure of atmospheric pressure at that location.
The standard atmospheric pressure at sea level can support a mercury column that rises to a height of 760 mmHg. This value is often used as the reference point for atmospheric pressure, and it is referred to as one standard atmosphere (1 atm).
Converting Pressure Units
In the world of science and engineering, pressure can be expressed in various units, including pascals (Pa), kilopascals (kPa), atmospheres (atm), and millimeters of mercury (mmHg). To work with pressure in different units, it’s essential to understand how they relate to each other. Here are some common pressure units and their conversions:
- 1 standard atmosphere (1 atm) is approximately equal to 101.3 kilopascals (kPa).
- 1 kilopascal (kPa) is equivalent to 7.5006 millimeters of mercury (mmHg).
Calculating Pressure in mmHg
To calculate pressure in mmHg, you can use the following formula:
Pressure (mmHg)=Pressure (Pa)1 mmHg≈133.3224 PaPressure (mmHg)=1 mmHg≈133.3224 PaPressure (Pa)
Here, “Pressure (Pa)” represents the pressure in pascals, which is the SI unit for pressure. Before using this formula, convert other pressure units, such as kilopascals (kPa) or atmospheres (atm), to pascals.
Let’s walk through an example to illustrate how to calculate pressure in mmHg:
Example: Suppose you have a pressure of 80 kPa, and you want to convert it to mmHg.
First, convert the pressure to pascals: 80 kPa=80,000 Pa80 kPa=80,000 Pa
Now, use the formula to convert to mmHg: Pressure (mmHg)=80,000 Pa133.3224 Pa/mmHgPressure (mmHg)=133.3224 Pa/mmHg80,000 Pa
Calculate the pressure in mmHg: Pressure (mmHg)≈600.57 mmHgPressure (mmHg)≈600.57 mmHg
So, a pressure of 80 kPa is approximately equal to 600.57 mmHg.
Practical Applications of mmHg
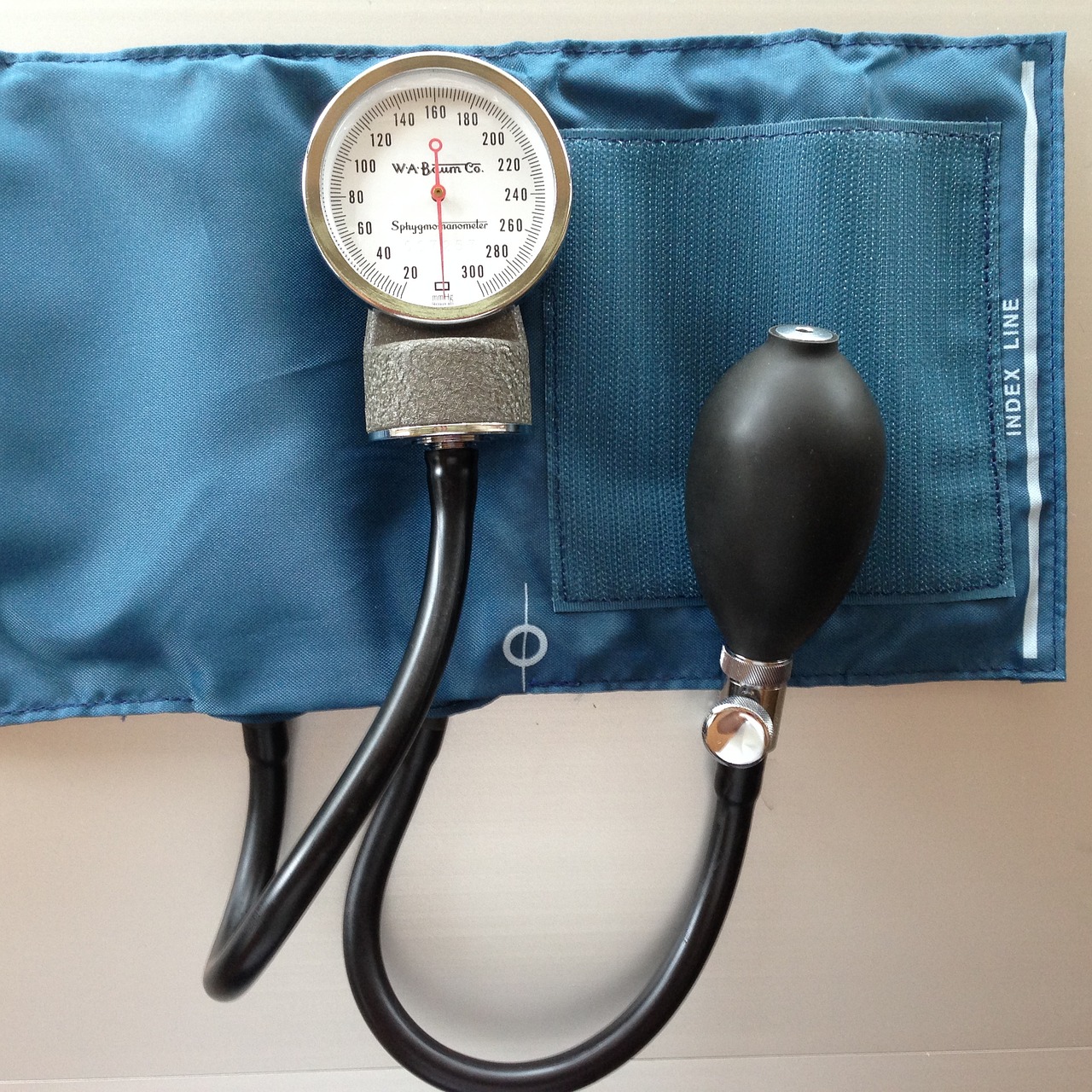
Millimeters of mercury (mmHg) is a unit commonly used in the field of medicine, particularly in the measurement of blood pressure. When a healthcare professional measures your blood pressure, they express it as two values: systolic and diastolic pressure. These values are typically measured in mmHg.
Systolic Pressure: This is the higher of the two numbers and represents the pressure in your arteries when your heart beats or contracts.
Diastolic Pressure: The lower number represents the pressure in your arteries when your heart is at rest between beats.
For example, a blood pressure reading of “120/80 mmHg” indicates that the systolic pressure is 120 mmHg, and the diastolic pressure is 80 mmHg. Blood pressure is a critical indicator of cardiovascular health, and monitoring it is essential for diagnosing and managing various medical conditions.
In summary, millimeters of mercury (mmHg) is a valuable unit for measuring pressure, particularly in medical applications like blood pressure monitoring. Understanding how to convert between different pressure units, such as pascals and atmospheres, allows us to work with pressure measurements effectively. Whether you’re in the realm of science, engineering, or healthcare, a grasp of mmHg and its conversions is essential for accurate and informed decision-making in your field.