For centuries, the engine of global industry has been powered by a simple, extractive principle: dig, refine, and burn. The combustion of fossil fuels has built our modern world, but it has come at an unsustainable cost—climate change, resource depletion, and environmental degradation. This paradigm has reached its logical conclusion. In its place, a new, restorative economic model is emerging: the bioeconomy. Encompassing everything from sustainable agriculture and biobased products to bioenergy, the bioeconomy leverages biological resources, processes, and principles to produce the food, materials, and energy our society needs.
But a crucial question remains: how do we efficiently transform raw, often complex biomass—from dedicated crops and agricultural waste to algae and engineered microbes—into the sophisticated, high-value products that underpin a modern society? The bridge between the potential of biology and the reality of the market is built by a transformative and multidisciplinary discipline: Advanced Bioprocess Engineering.
Moving far beyond traditional fermentation, advanced bioprocess engineering is the sophisticated engine that converts groundbreaking biological discoveries into scalable, economically viable, and sustainable industrial processes. It is the critical, often unsung, link between lab-scale promise and global commercial impact, turning the vision of a circular bioeconomy into a tangible reality.
From Art to Science: The Pillars of Advanced Bioprocess Engineering
The term “advanced” signifies a fundamental shift from an empirical craft to a precise, data-driven science. Where traditional bioprocessing might rely on established recipes and reactive controls, advanced bioprocess engineering is proactive, predictive, and integrated. It rests on several interconnected pillars:
1. The Cellular Architect: Systems Biology and Metabolic Engineering
Before a single bioreactor is designed, the microbial cell itself must be conceived and optimized as a production unit. This is the realm of systems biology and metabolic engineering. Systems biology provides a holistic, “omics”-driven view (genomics, transcriptomics, proteomics, metabolomics) of the intricate networks within a cell. It allows engineers to understand the system as a whole, rather than as a collection of isolated parts.
Metabolic engineering is the applied art of rewiring these cellular networks. Think of it as reprogramming a microorganism’s internal software to turn it into a highly efficient cell factory. Using tools like CRISPR-Cas9, engineers can knockout non-essential genes, introduce new metabolic pathways from other organisms, and fine-tune gene expression to direct the cell’s resources toward overproducing a specific target compound. This could be a biofuel like isobutanol, a platform chemical like succinic acid, or a therapeutic protein. The goal is maximal yield, high productivity, and minimal byproduct formation, setting the stage for an efficient and economical large-scale process.
2. The Performance Stage: Advanced Bioreactor Design and Process Intensification
The bioreactor is the stage where the engineered cellular performance unfolds. Advanced designs have moved far beyond the simple stirred-tank reactor of the past. The focus is on Process Intensification—doing more with less, in a smaller footprint, with greater efficiency.
Continuous Bioprocessing: Traditional batch fermentation is akin to cooking a meal, cleaning the pot, and starting again. Continuous processing, however, operates like a steady assembly line, with fresh nutrients fed in and product continuously harvested. This leads to significantly higher volumetric productivity, more consistent product quality, reduced reactor size, and lower operational costs. While well-established in traditional chemical engineering, its application to sensitive biological systems is a hallmark of advanced bioprocessing.
Single-Use Bioreactors: Particularly dominant in pharmaceutical and high-value chemical production, these disposable, pre-sterilized plastic bags eliminate the risk of cross-contamination and the need for costly and energy-intensive cleaning-in-place systems. They offer unparalleled flexibility, drastically reduce downtime between batches, and accelerate process development.
Multiphase and Membrane Bioreactors: For processes where the product inhibits the microbes or is volatile, integrated systems are key. Membrane bioreactors, for example, allow for the continuous separation of products from the fermentation broth, driving equilibrium-limited reactions to completion and maintaining a healthy culture for longer periods.
3. The Unsung Hero: Integration and Innovation in Downstream Processing
The biological transformation inside the bioreactor—the “upstream” process—is only half the battle. The subsequent separation, purification, and polishing of the target molecule from a complex broth containing cells, media components, and byproducts is known as downstream processing. This stage is often the bottleneck and can constitute a staggering 50-80% of the total production cost in biomanufacturing.
Advanced bioprocess engineering attacks this challenge through integration and innovation. Instead of treating upstream and downstream as separate silos, they are designed in concert. An upstream engineer might select a microbial host not only for high yield but also for its ability to secrete the product into the broth, simplifying initial recovery. Advanced separation techniques, such as expanded bed adsorption, continuous centrifugation, tangential flow filtration, and simulated moving bed chromatography, are employed to reduce energy consumption, solvent use, and waste generation. The ultimate goal is a seamless, continuous, and efficient pipeline from raw material to pure product.
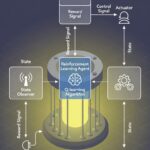
4. The Digital Nervous System: Process Analytical Technology (PAT) and AI
Perhaps the most significant leap forward in recent years is the digital transformation of bioprocessing. The FDA-initiated framework of Process Analytical Technology (PAT) embodies the shift from “Quality by Testing” (checking the product at the end) to “Quality by Design” (building quality into the process from the start).
PAT involves a suite of in-line, on-line, and at-line sensors that monitor critical process parameters (CPPs) in real-time. These go beyond standard pH and dissolved oxygen probes to include sophisticated spectrometers (NIR, Raman) that can provide real-time concentrations of substrates, metabolites, and even the product itself. This creates a rich, dynamic data stream.
When this data stream is fed into Artificial Intelligence (AI) and Machine Learning (ML) models, the process becomes truly intelligent. AI algorithms can analyze historical and real-time data to identify complex, non-linear relationships that are invisible to the human eye. They can predict optimal feeding strategies, forecast cell growth trajectories, and, most importantly, anticipate process deviations or failures before they occur, allowing for proactive control. This moves biomanufacturing from a reactive, recipe-based operation to a predictive, precisely controlled, and self-optimizing system.
Driving the Bioeconomy: From Concept to Tangible Applications
The impact of these advanced techniques is no longer theoretical; it is being felt across multiple sectors, creating tangible products and displacing fossil-based alternatives.
Sustainable Aviation Fuel (SAF): The aviation industry, difficult to electrify, urgently needs drop-in biofuels. Companies like LanzaJet are using engineered microbes to convert industrial off-gases or agricultural residues into ethanol and then, via catalytic processes, into jet fuel. Advanced bioprocess engineering is essential to scale these fermentation processes to the monumental volumes required by global aviation, all while ensuring the process is robust and cost-competitive.
Biobased Chemicals and Materials: The molecules for our everyday products can now be brewed, not drilled. Companies are producing the building blocks for plastics (e.g., Polylactic Acid (PLA) and Polyhydroxyalkanoates (PHAs)), nylons, and synthetic textiles from plant sugars instead of petroleum. This not only reduces carbon emissions but also creates biodegradable and compostable products, directly addressing the global plastic pollution crisis.
Precision Fermentation for Food: A revolution is underway in how we produce food. The alternative protein sector, including companies like Perfect Day (producing animal-free whey protein) and those cultivating real meat from animal cells, relies entirely on advanced bioprocessing. Growing mammalian cells or engineering Trichoderma fungi to secrete bovine proteins requires ultra-pure, meticulously controlled environments that are the hallmark of this discipline. The scalability and cost-reduction of these processes are key to their market success.
Waste Valorization and the Circular Economy: The most profound expression of the bioeconomy is closing the loop on waste streams. Advanced bioprocesses are being designed to convert food waste, wastewater sludge, and captured carbon dioxide into valuable products. This includes anaerobic digestion to produce biogas, microbial conversion of CO2 into bioplastics, and fermentation of food waste into lactic acid for PLA production. This transforms linear “take-make-dispose” models into circular, regenerative systems.
Navigating the Challenges: The Road Ahead
Despite its immense promise, the field is not without significant hurdles. The inherent complexity and variability of biomass feedstocks pose a major challenge to consistent processing. The capital expenditure for building first-of-their-kind commercial-scale bioprocessing plants is high, representing a significant investment risk. Furthermore, there is a pressing need for a new generation of skilled workers who can operate at the intersection of biology, chemical engineering, data science, and robotics.
The path forward requires a concerted effort from industry, academia, and government:
Further Deep Integration: A “bioprocess-led” approach to strain design, where the microbe is engineered with the constraints and opportunities of the entire manufacturing process in mind from the outset.
Robust Scale-Down Models: Developing small-scale (e.g., microtiter plates, mini-bioreactors) systems that accurately mimic the hydrodynamics and mass transfer effects of large-scale production tanks. This allows for high-throughput screening and de-risking of scale-up, which remains a major bottleneck.
Supportive Policy Frameworks: Government incentives, carbon pricing mechanisms, and standardized lifecycle assessments that recognize the superior environmental benefits of biobased products are essential to level the playing field with entrenched fossil-based incumbents.
Conclusion
Advanced Bioprocess Engineering is no longer a niche academic field; it is the indispensable industrial discipline for building a sustainable, resilient, and prosperous 21st century. It is the tangible manifestation of our growing mastery over biological systems for the benefit of society and the planet. By intelligently harnessing the transformative power of biology and marrying it with cutting-edge engineering, digital intelligence, and circular principles, we are learning to manufacture the goods our civilization needs in harmony with the natural world. It is, without exaggeration, the key that unlocks the full potential of the bioeconomy, transforming it from a promising concept into an operational reality that can fuel, feed, and furnish our future.