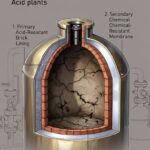
Scaling a process from the lab bench to full commercial production is a complex journey involving numerous technical, financial, and logistical considerations. This evolution—from proof of concept to continuous manufacturing—requires a deep understanding of engineering principles, regulatory compliance, and operational management. To navigate this path successfully, it’s essential to understand the terminology that defines each stage. In this article, we explore the critical phases of industrial scale-up and the associated language used across industries like chemical processing, pharmaceuticals, energy, food, and advanced materials.
1. Lab Scale: Where Innovation Begins
The journey to commercialization often begins at the lab scale. Here, scientists focus on discovery, reaction mechanisms, and small-scale proof-of-concept studies. The operations are conducted using benchtop reactors and analytical instruments, and while the quantities involved are small, the insights are substantial.
Key Terms:
Proof of Concept (PoC): The initial demonstration that a concept or technology is feasible.
Bench Scale: Experiments carried out on laboratory workbenches, often involving milliliters to a few liters of material.
Feasibility Study: An assessment of the practicality of a proposed process or product.
Reaction Optimization: Fine-tuning reaction conditions such as temperature, pressure, and reagent ratios to maximize yield and selectivity.
Lab-scale activities help determine whether the idea is worth investing in for further development. However, at this point, economic viability and scalability are usually not yet addressed in full.
2. Pilot Scale: Bridging Science and Engineering
Once a process is proven at the lab scale, it transitions into the pilot scale. A pilot plant is a scaled-down version of the eventual manufacturing facility, designed to test process performance under realistic conditions. It’s a critical stage that simulates industrial operations while allowing for the adjustment of parameters and identification of potential issues before committing to expensive full-scale infrastructure.
Key Terms:
Pilot Plant: A facility that mimics the operations of a commercial plant at a reduced scale.
Process Development Unit (PDU): A term often used interchangeably with pilot plant, particularly in the energy and chemical sectors.
Process Validation: A series of activities to ensure that a process operates within defined parameters and consistently produces quality output.
Design of Experiments (DoE): A statistical approach to studying how multiple variables affect a process and identifying optimal conditions.
Process Intensification: Techniques aimed at making chemical processes more efficient, compact, and sustainable.
Technology Readiness Level (TRL): A metric used to assess how close a technology is to full deployment. Pilot scale typically falls around TRL 5–6.
The goal at this stage is to gather detailed operational data, validate the process under scaled conditions, and begin outlining engineering designs for the next phase.
3. Demonstration Scale: Proving Viability at a Larger Scale
Before going fully commercial, companies often build a demonstration plant—a near-commercial facility that operates under conditions and volumes closer to the intended production scale. The demonstration scale is critical for derisking investment and proving the technology’s robustness to stakeholders, investors, and regulatory agencies.
Key Terms:
Demo Plant: A near-commercial setup that proves the operability and economics of a process under extended run times.
Pre-Commercial Scale: A transitional phase used to test the process in real-world conditions without full commercial risk.
Proof of Scale: Demonstrates that a process scaled from lab and pilot can perform consistently at larger volumes.
Extended Operations Testing: Running the plant for prolonged periods to observe system reliability and long-term behavior.
Process Simulation: The use of software tools to model and optimize process flows and energy/material balances.
Demonstration plants are often the final proving ground before full-scale investment. They provide invaluable insights into potential operational bottlenecks, yield variations, or equipment constraints.
4. Commercial Scale: Reaching Full Production
At the commercial scale, the process is fully operational, producing materials at the intended nameplate capacity and under stringent quality and safety controls. This stage requires deep integration of engineering, logistics, finance, and management systems to achieve production targets cost-effectively and sustainably.
Key Terms:
Full Scale Production: The operation of the plant at design capacity, typically the goal of the commercialization effort.
Commercialization: The process of introducing a new product or process into the market at industrial scale.
Nameplate Capacity: The maximum output a plant is designed to produce under optimal conditions.
Ramp-Up Period: The gradual increase of production output as systems stabilize and workforce becomes accustomed to operations.
Plant Commissioning: A systematic process of ensuring all systems and components of the plant are designed, installed, and tested according to operational requirements.
Operational Qualification (OQ): Verifying that systems operate according to defined parameters under simulated conditions.
Performance Qualification (PQ): Confirming that the plant consistently performs as required under real operating conditions.
During this phase, the focus shifts to supply chain integration, operational efficiency, product quality, and regulatory compliance.
5. Supporting Concepts in Scale-Up
Each phase in the scale-up journey is supported by cross-cutting principles and practices that ensure safety, quality, and operational excellence.
Key Concepts:
Scale-Up Factor
This is the ratio between the lab, pilot, or demo process and the final commercial scale. Understanding and managing this factor is vital to maintaining consistency and yield.
Tech Transfer
Short for Technology Transfer, this process involves the formal handover of process knowledge from R&D or pilot teams to the commercial manufacturing group. It includes SOPs, process parameters, raw material specifications, and risk assessments.
Process Safety Management (PSM)
As volumes increase, so does the risk. PSM involves hazard identification, risk mitigation, emergency planning, and compliance with safety standards to prevent catastrophic failures.
Regulatory Compliance
Depending on the industry, processes must meet specific regulatory standards (e.g., FDA for pharmaceuticals, EPA for environmental compliance, OSHA for worker safety). Compliance must be built into the process from the pilot stage onward.
Supply Chain Integration
Commercial success is not just about making a product; it’s about delivering it reliably and cost-effectively. This includes sourcing raw materials, managing logistics, and aligning production schedules with market demand.
Conclusion: Navigating the Scale-Up Journey
Scaling from pilot plant to commercial production is far more than simply “making more product.” It’s a multidisciplinary journey that requires collaboration between chemists, engineers, business strategists, and regulatory experts. Understanding the key terms and milestones associated with each phase helps align teams, manage expectations, and reduce risk at every step.
Each stage—lab, pilot, demonstration, and commercial—plays a vital role in validating not just the science, but the economics and practicality of the process. And while the path to commercialization is rarely linear, a strong grasp of industry language can serve as a compass, guiding innovations from concept to market impact.